What is isoelectric focusing?
A powerful tool for the analysis of native protein mixtures!
Electrophoresis is a technique that enables separation and analysis of charged molecules in an electric field.
A new service for potential newcomers is a free examination of protein samples using high-resolution electrophoresis (isoelectric focusing).
Send us samples with a brief description of the material and we will send you the results within 1 week.
All electrophoretic separations of proteins make use of the fact that each protein has a net charge that varies with the pH of its environment. This net charge represents the sum of positive and negative charges on the surface of a protein. When passing from a very low pH to a very high pH, the net charge changes in a continuous manner from plus to minus. At a certain well defined pH, the net charge equals zero. This point is called the isoelectric point.
As mentioned above, electrofocusing utilizes a stationary and stable pH gradient produced by a chemically synthesized carrier ampholyte. lf a protein is introduced into such a gradient at a pH lower than its own pl, it will obtain a positive net charge and under the influence of the electric field, will move towards the cathode. On approaching its pl, the net charge will gradually decrease and finally, when it reaches the pl position, the net charge will equal zero and migration will cease. lf the protein is placed at a pH higher than its pl, the net charge will be negative and the protein will move towards the anode. ln the same manner as above it will be trapped at the pl position.
Thus, irrespective of where a protein sample is introduced into the pH gradient, each protein will end up as a narrow zone at a position corresponding to its pl. lf a focused protein tries to escape from the pl position, e.g. by diffusion, it will obtain a net charge that forces it back to its pl.
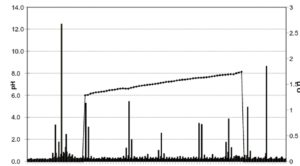
All this needs a stable stationary pH gradient. Such a gradient is brought about by using a mixture of specially designed amphoteric substances, so-called carrier ampholytes. Like proteins, these substances possess net charges that vary with pH and have different pls.
From a general point of view it is desirable to keep the experimental time as short as possible. ln all electrophoretic techniques the experimental time depends on the applied voltage. Evolution of heat is invariably connected to electrophoretic techniques and the higher the power used the more heatwill be produced. ln reality it is the capacity of the cooling system that limits the applicable power and thus the highest voltage and consequently the minimum experimental time.
The formation of the pH gradient is accompanied by a pronounced drop in conductivity. A power supply that can provide only constant current or constant voltage is unable to maintain an optimal voltage under such circumstances. However, if power is kept constant and matches the cooling capacity, the voltage will be as high as possible at any given conductivity. This in turn will keep the experimental time as short as possible.
Another factor that affects the experimentaltime is the pH range employed. A wide pH range will produce a steep pH gradient that will keep the proteins highly charged even in the close vicinity of their respective pls, whereas the shallow gradient resulting from a narrow pH-range induces a low net charge of the proteins rather distant from their pls.
From the experimental time point of view this means that the narrower the pH range used, the longer the time needed for electrofocusing experiments. Of course, the focusing time will vary between individual proteins depending on their respective pH/mobility curves. For most proteins, however, 1.5 hours is satisfactory when employing the wide pH range of 3.5-9.5.
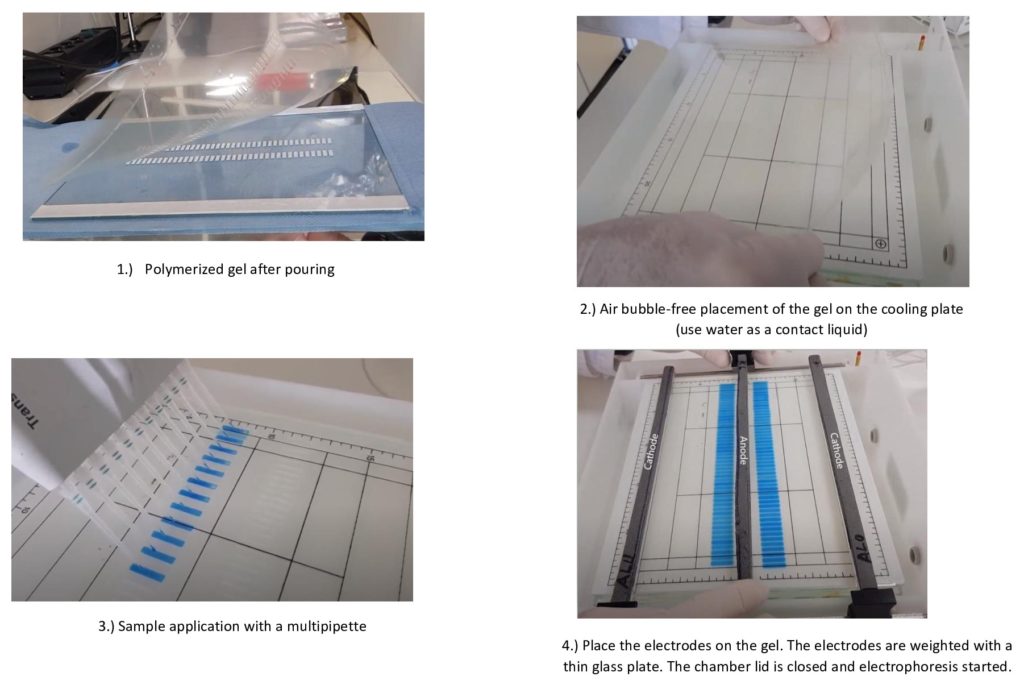
The electrophoresis of the proteins mainly takes place in a matrix such as agarose, acrylamide or the newly developed matrix ProPhly Air.
Uses of IEF
Analytical IEF can be used to assess the complexity of protein extracts and to identify components of interest. However, the great resolving power of IEF makes it ideal for detection of microheterogeneity in purified proteins. Enzymologists have used IEF to detect isoforms of purified enzymes and protein biochemists have demonstrated ‘multiple forms’ of many proteins which appear homogeneous using other biochemical techniques (including other electrophoretic methods). Focusing is used for the diagnosis and study of some genetically transmitted disorders. The basis of the slight differences in the pI of isoforms as revealed by IEF is often post-translational modification of a protein, i.e. a single primary structure which is modified after synthesis. This is frequently due to variation in the glycosylation of a protein and, in particular, the number of sialic acid residues present. Immunochemists often use IEF for the analysis of a variety of antigens and preparations. The technique is very useful when combined with immunoblotting; this enables the precise antigen-binding profile of an antiserum or monoclonal antibody to be established.
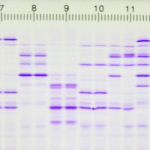
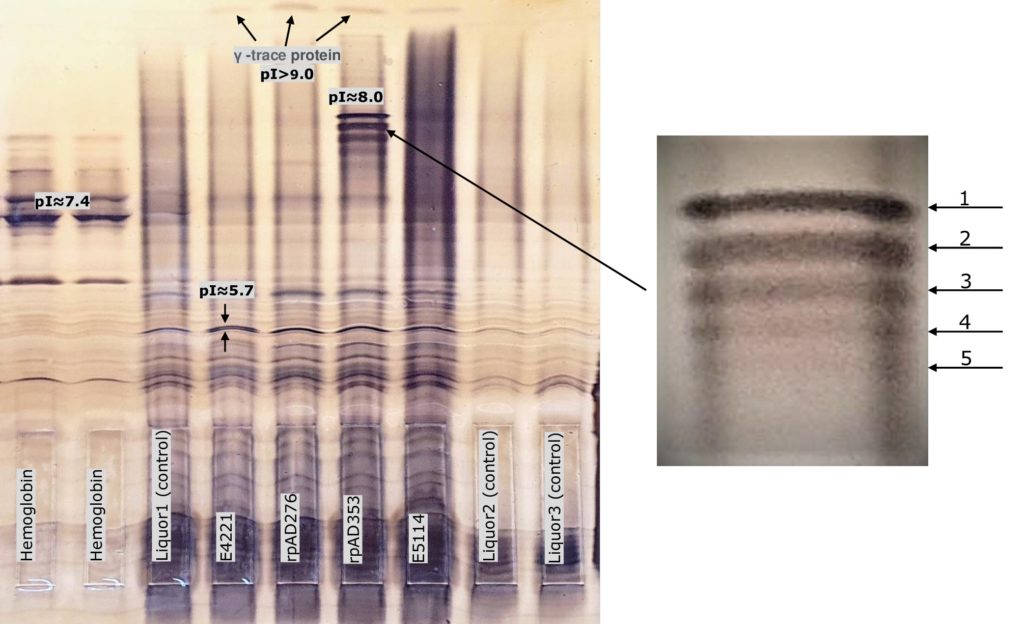
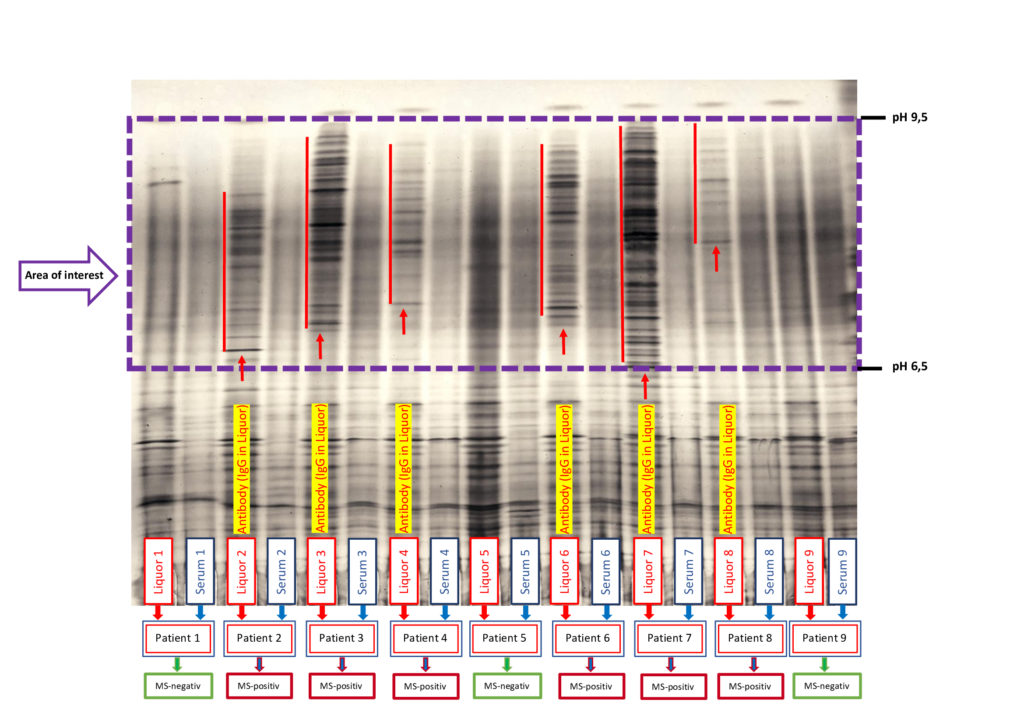
Multiple sclerosis analysis with determination of the immunoglobulins (IgG) in the CSF
Discovery in Alzheimer’s research with the help of ProPhyl Air
Aß-oligomer (pentamer) protein was detected in cerebrospinal fluid of Alzheimer’s samples
CSF examination service
ProTec Bioseparation offers cost-effective examinations of MS (IgG) and Alzheimer’s samples (liquor) to support research laboratories in their analyses. The aim of this service is to provide an initial orientation for neurodegenerative diseases and help confirm suspected cases (e.g. multiple sclerosis).
To use our prompt service, please send us the most up-to-date sample material possible (CSF) and, for suspected cases of MS, the corresponding serum. (contact: m.demharter@protec-biosep.de)
Carrier ampholytes
Sepalyte™ carrier ampholyte is the trade name of a 40% v/v mixture of such carrier ampholytes with pl values tightly arranged over a specified pH range and is normally used at a concentation of 2%, which allows them to dictate the pH gradient. lf such a mixture is subjected to an electrical field, the carrier ampholytes will automatically arrange themselves in the order of their pls so that those with the lowest pls will end up at the anode and those with the highest pls will be found at the cathode. When the pH gradient is fully established, the net charges of all the carrier ampholytes will be zero and the gradient becomes stationary. As with focused proteins, diffusion of carrier ampholytes is counteracted by the electrical field.
This, together with the buffering properties of carrier ampholytes, results in a stable pH gradient suitable for isoelectric focusing experiments.