Tomato
ACP – (Acidphoshatase)
0.8 M Tris-formate pH 9.0
48.4 g Tris (q.c.w. Sigma T-8524) in 400 ml distilled water. Stir until fully dissolved. Adjust the pH to 9.0 with formic acid (q.c.w. Sigma F-0507). Bring to a final volume of 500 ml with distilled water. Store in the refrigerator.
25 % v/v Glycerol
Bring 78 grams (= 62.5 ml) glycerol (q.c.w. Sigma G-7757) and 200 ml distilled water in a beaker. Mix well and bring to a final volume of 250 ml with distilled water. Store in the refrigerator
Acrylamide/bis acrylamide 29 :1 solution
Ready to hand acrylamide solution (q.c.w. Sigma A-3574). Store in refrigerator.
0.1N Sodium hydroxide
4.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 1000 ml distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature.
0,1% w/v Riboflavin
0.05 g riboflavin (q.c.w. Sigma R-0508) in 50 ml 0.1 N sodium hydroxide. Store in the refrigerator for up to 2 weeks
10% w/v Ammonium persulfate
0.5 g ammonium persulfate (q.c.w. Sigma A- 3678) in 5.0 ml distilled water. Store in refrigerator for no more than one week.
Cathode solution 0.6 M glycine
11.25 g glycine (q.c.w. Sigma G-7126) in 250 ml distilled water. Add a trace (tip of a small spatula) of bromphenol blue (q.c.w. Sigma B-8026). Store in refrigerator.
Anode solution 0.175 M Tris-formate pH 9.0
40 ml of 0.8 M Tris-formic acid pH 9.0 plus 140 ml distilled water. Store in refrigerator
25% v/v Polyethelene glycol (PEG)
Bring 250 ml polyethylene glycol (q.c.w. Sigma P-3265) and 500 ml distilled water in a beaker. Stir until PEG is fully dissolved. Bring to a final volume of 1000 ml with distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store in refrigerator. PEG can be reused many times.
0.1 M Sodium acetate pH 5.0
Bring 16.4 g sodium acetate (q.c.w. Sigma S-8750) in 1800 ml distilled water. Stir until fully dissolved. Adjust the pH to 5.0 with acetic acid (q.c.w. BDH 27013). Bring to a final volume of 2000 ml with distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature.
4 M Magnesium chloride
Bring 203-g magnesium chloride hexahydrate (q.c.w. Sigma M-0250) and 150 ml distilled water in a beaker. Stir until fully dissolved and bring to a final volume of 250 ml. Store at room temperature.
10 % v/v Glycerol solution
Bring 125 grams (= 100 ml) glycerol (q.c.w. Sigma G-7757) and 800 ml distilled water in a beaker. Mix well and bring to a final volume of 1000 ml with distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store in the refrigerator. The 10 % glycerol solution can be reused many times.
TEMED
Ready to use TEMED Sigma (T-9281).
PAGE solution
Combine 312 ml of 25% glycerol with 1 Liter of 0.8 M Tris-formate pH 9.0. Store at room temperature for up to two weeks.
Gel preparation
26.0 ml PAGE solution: 25% glycerol and 0.8 M Tris-formate pH 9.0
10.0 ml Acrylamide/bis acrylamide 29:1 solution
0.06 ml TEMED 0.48 ml 10 % w/v ammonium persulfate
Add the above reagents and swirl to mix. Pour the gel according to the flap technique and allow polymerizing for at least 30 minutes. Store the gels in a sealed bag in the refrigerator for up to 1 week.
Sample preparation
Single first leaf punches(using the cork borer # 3 , diameter 7 mm) from soil germinated seeds (12-15) days old are placed into a 96 well micro plate. To each well, 50 µl water (add trace bromophenol blue as tracking dye) is added. See to it that the confetti-like punchouts are on the bottom of the wells. The samples are frozen and homogenized using the Terminator for 3 minutes and centrifuged for 10 minutes at 3000 – 3500 rpm. at 10°C (or at room temperature)
Electrophoresis
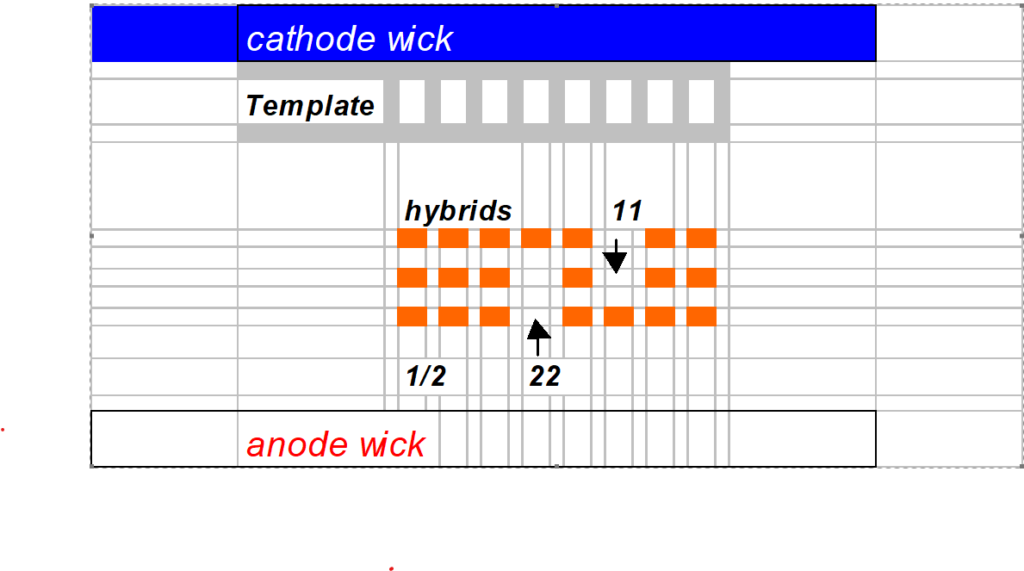
Turn the cooling supply on and set at a temperature of ± 15 ° C. Remove the gel from the glass plates. Clean the back of the gel with methanol/ethanol. Place the gel onto the cooling plate with several ml of water. The gel is divided into three parts by spacing the electrodes evenly across the gel, alternating cathode (black electrode) anode (red electrode). Place the electrode plateau directly onto the gel making electrode lines in the gel. Blotting paper wicks (3x 12 x 260 mm) are used. Wet two cathode wicks with 0.6 M glycine (± 25ml) and two anode wicks with 0.175 M Tris-formate pH 9.0 (± 25 ml). Gently blot the wicks but keep them fairly wet. Place the cathode wicks onto the cathode prints in the gel and the anode wicks onto the anode prints. The 52 well-clear templates are positioned directly against the cathode wick. That is to say; no space between the wicks and templates. See cartoon in section “gel interpretation.
Sample application
Each well is filled with 15ml of supernatant. Place the electrode plateau on the wicks.
Prefocusing
Run 1: 1000 V–75 mA–10 W–45 min.
Run 2: 1000V-75mA-20W-60 min.
These settings are empiric. Follow the bromophenol blue marker line. Being at the anodal side for 5 minutes, indicates the end of the run.
After the gel has finished running, rinse the gel in an icy-cold 25% v/v PEG solution for 10 minutes. Wash away the PEG of the gel with water and then stain with ACP staining solution.
ACP staining
200 ml 0.1 M sodium acetate pH 5.0
0.32 ml 4 M MgCl2
0.32 g b- naphthyl acid phosphate (q.c.w. Sigma N-7375)
0.32 g Fast Garnet (q.c.w. Sigma F-8761)
Heat up the sodium acetate solution to ± 37° C. Add the reagents together and mix until fully dissolved and stain the gel at ± 37°C or room temperature until the bands can be clearly visualized. This might take more than one hour. Remove the stain and bring the gel in a 2 % glacial acetic acid solution for about 10 minutes and rinse with distilled water. Finally, remove the water/acetic acid mixture and submerge the gel in a 10 % v/v glycerol for 20 minutes. There are two ways to preserve the gels:
The sticky gel can be preserved using special (expensive!) cellophane sealing sheet (Pharmacia art # 80-1129-38) the gel can then be air-dried.
Using a sealing device can seal the gel after the gel has been air-dried.
Gel interpretation
No international enzyme classification is used.
The nearest band of interest from the anode is genotyped “11”
The farthest band of interest from the anode is genotyped “22”.
The hybrid is genotyped “1/2” in case that the female is 11 and the male 22.
The hybrid is genotyped “2/1” in case that the female is 22 and the male 11.
ProTec Bioseparation (Protein Electrophoresis)

