— Breakthrough —
Parkinson’s biomarkers can now be detected in CSF (cerebrospinal fluid)
CSF-protein analysis to aid in detection of neuroinflammatory diseases
The diagnosis of Parkinson’s relies on clinical symptoms, but the identification of CSF biomarkers could be the key to greater diagnostic and prognostic accuracy in tackling this disease.
https://www.linkedin.com/embed/feed/update/urn:li:share:7081189169581936640
Parkinson’s: an autoimmune disease?
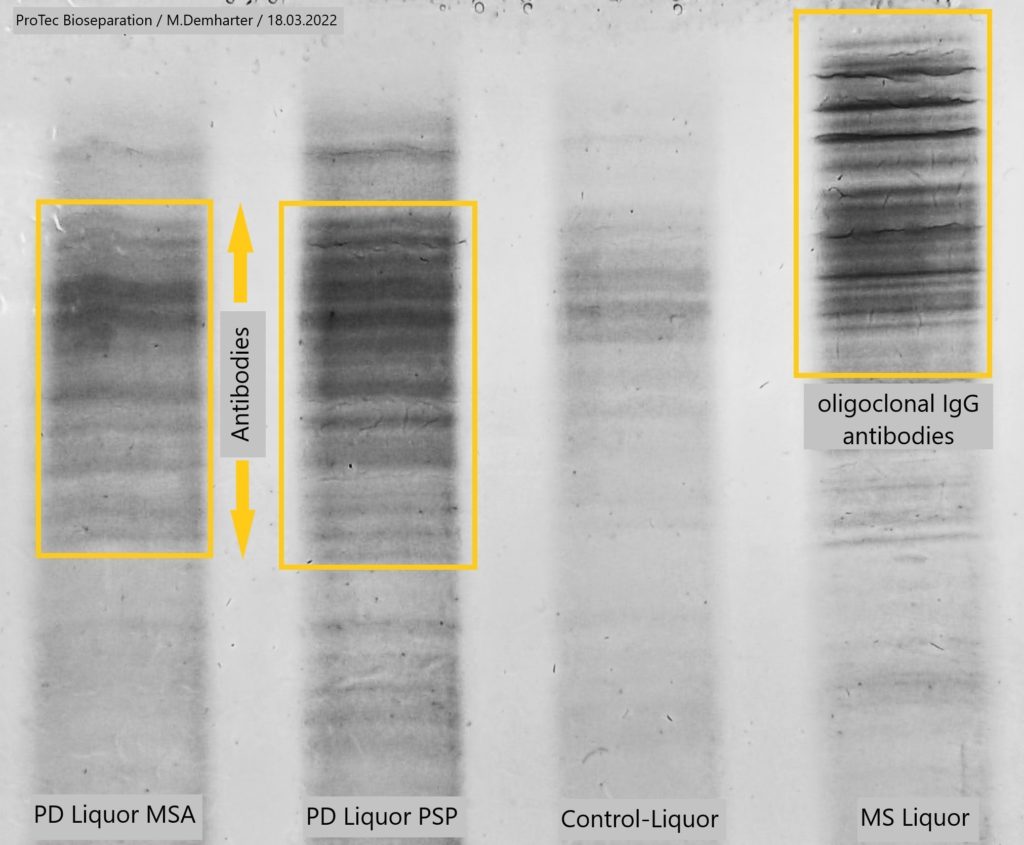
The antibodies detected for the first time in CSF using the HIEF method in patients with the Parkinson’s variant PSP (Progressive Supranuclear Palsy) support the following assumptions:
For decades, researchers and physicians alike have found that inflammation drives the changes in the brains of people with Parkinson’s disease. However, it is only in recent years that they have been able to understand that this inflammation is part of the cause of the progressive nature of Parkinson’s disease and not just a consequence of the disease.
A 2017 study led by Sette and Sulzer was the first to show that alpha-synuclein can act as a beacon for certain T cells, causing them to mistakenly attack brain cells and potentially contribute to the progression of Parkinson’s. This was the first direct evidence that autoimmunity could play a role in Parkinson’s disease.
Furthermore, research by Columbia University and the La Jolla Institute for Allergy and Immunology in the United States has likewise found evidence of the role that autoimmunity plays in Parkinson’s disease. The researchers claim that two toxic fragments of the protein that appears in the brain cells of people with Parkinson’s, alpha-synuclein, can activate T cells.
According to the researchers, the autoimmunity of Parkinson’s arises when neurons cannot get rid of abnormal alpha-synuclein. This protein begins to accumulate because the process of protein recycling decreases with age and certain diseases. Since the immune system had not identified it, it was considered a pathogen to be attacked.
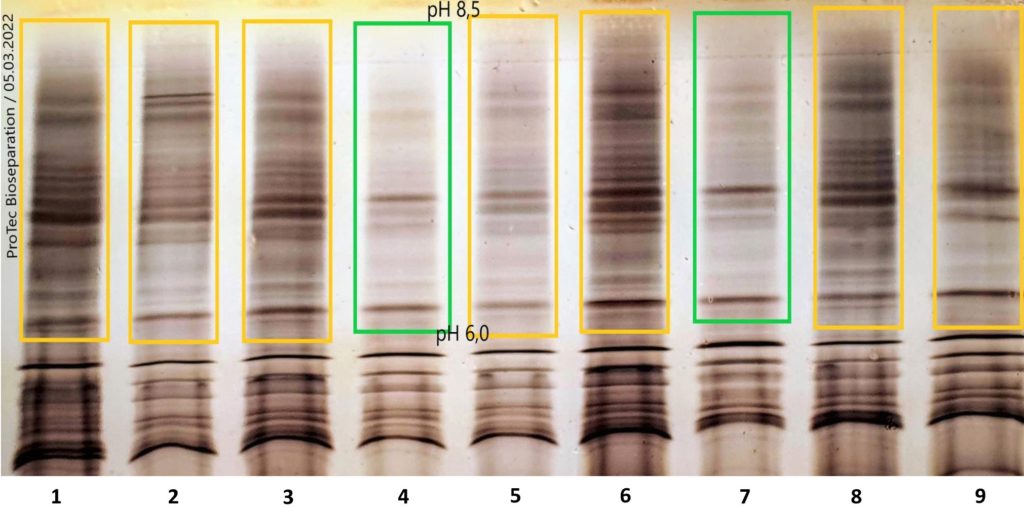
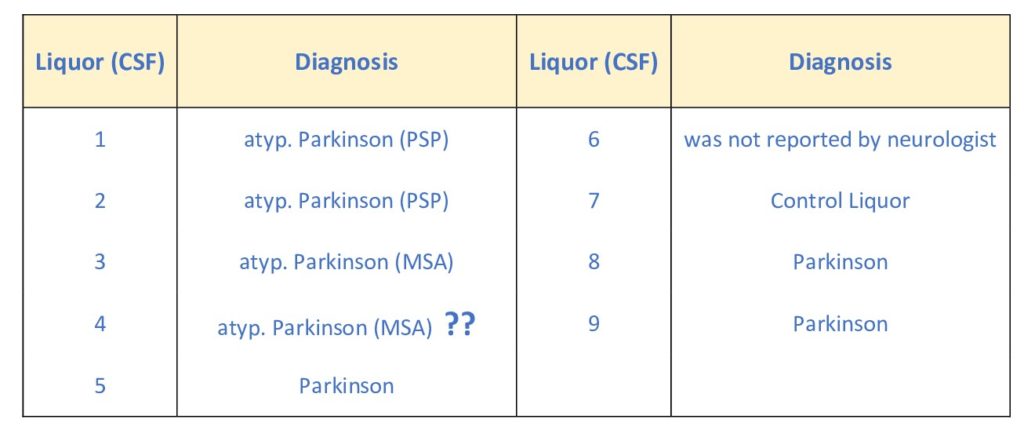
Samples:
Universitätsmedizin der Johannes Gutenberg-Universität Mainz
Klinik und Poliklinik für Neurologie
Sektion für Bewegungsstörungen und Neurostimulation
Forschungszentrum Translationale Neurowisssenschaften (FTN)
Rhein-Main-Neuronetzwerk (rmn2)
Parkinson’s and Multiple Sclerosis
Is there a link?
A team of researchers has discovered new evidence that Parkinson’s disease may have an infectious or autoimmune origin. As in multiple sclerosis, antibodies are the result of inflammation in the brain in Parkinson’s disease.
The genetic variant associated with Parkinson’s disease is located in the same region as the one associated with multiple sclerosis. The cause is not yet fully understood, but one possibility could be a misfolded and therefore toxic alpha-synuclein that triggers the inflammatory process. The “gut-brain axis” hypothesis in PD holds that alterations in the gut microbiota may favor α-Syn aggregation and are responsible for an inflammatory response in the periphery, which includes increased cytokine levels and activated T cells.
Does Parkinson’s cause brain inflammation?
It is well documented (Braak’s Hypothesis) that autopsied brains from patients with Parkinson’s disease (PD) show evidence of inflammation within the region of the brain thought to be responsible for PD.
Check out the animation below!
Animation created by Susie Yun, MA through collaboration with Drs. Ted and Valina Dawson.
Logical consequence: the presence of antibodies in the CSF of PD patients.
However, such antibodies have not so far been found!
What is the HIEF technique?
HIEF stands for Hydrophobic interaction isoelectric focusing
(For detailed information on HIEF technology, please go to: www.protec-biosep.de)
HIEF is a highly sensitive method capable of detecting these PD antibodies at an early stage and thus enabling early application of appropriate therapies. The method could be also highly beneficial in pharmaceutical research as a tool for tracking disease progression and the impact of medication at the trial stage.
What is Parkinson’s disease?
Parkinson’s disease (PD) is a movement disorder of the nervous system that worsens over time. As nerve cells (neurons) in parts of the brain weaken or are damaged or die, people may begin to notice problems with movement, tremor, stiffness in the limbs or the trunk of the body, or impaired balance. As these symptoms become more obvious, people may have difficulty walking, talking, or completing other simple tasks. Not everyone with one or more of these symptoms has PD, as the symptoms appear in other diseases as well.
No cure for PD exists today, but research is ongoing and medications or surgery can often provide substantial improvement with motor symptoms.
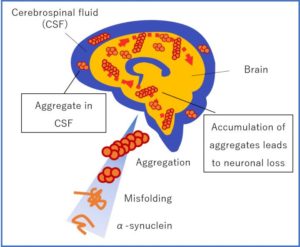
Degenerate α-synuclein causes the disease
Protein aggregates have been observed in the nerve tissue of patients with Parkinson’s disease which consist of individual components (monomers) of the protein alpha-synuclein. These assemble into what are referred to as amyloid fibrils. Similar deposits are also found in the case of other neurodegenerative diseases such as Alzheimer’s. Researchers are looking for approaches to prevent fibril formation and potentially cure the diseases.
Aggregates of amyloid proteins characterize many neurodegenerative disorders including Alzheimer’s disease (AD) and Parkinson’s disease (PD). Formation of pathological inclusions occurs by a multistep process including the misfolding of normal soluble proteins and their association into higher-order oligomers, followed by their assembly into amyloid fibrils (figure 5) that form disease-specific inclusions.

Native α-synuclein
H. Lundbeck A/S
Denmark

Degenerate α-synuclein
H. Lundbeck A/S
Denmark
Large amounts of insoluble aggregated fibrils (left+right), caused by a degenerative α-synuclein can be found on the application point on a gel. Causally responsible for Parkinson’s disease.
The size of the fibrils can be well over 2 Megadalton (2MDa) and thus no longer accessible for electrophoresis
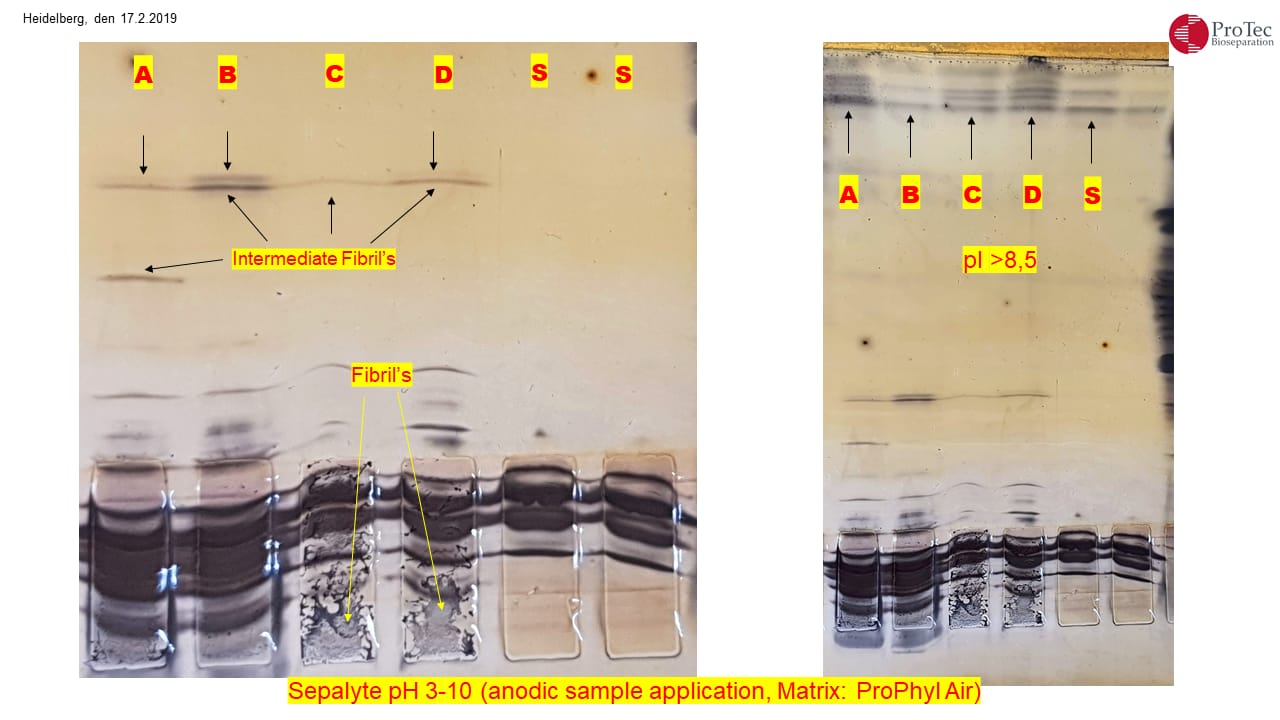
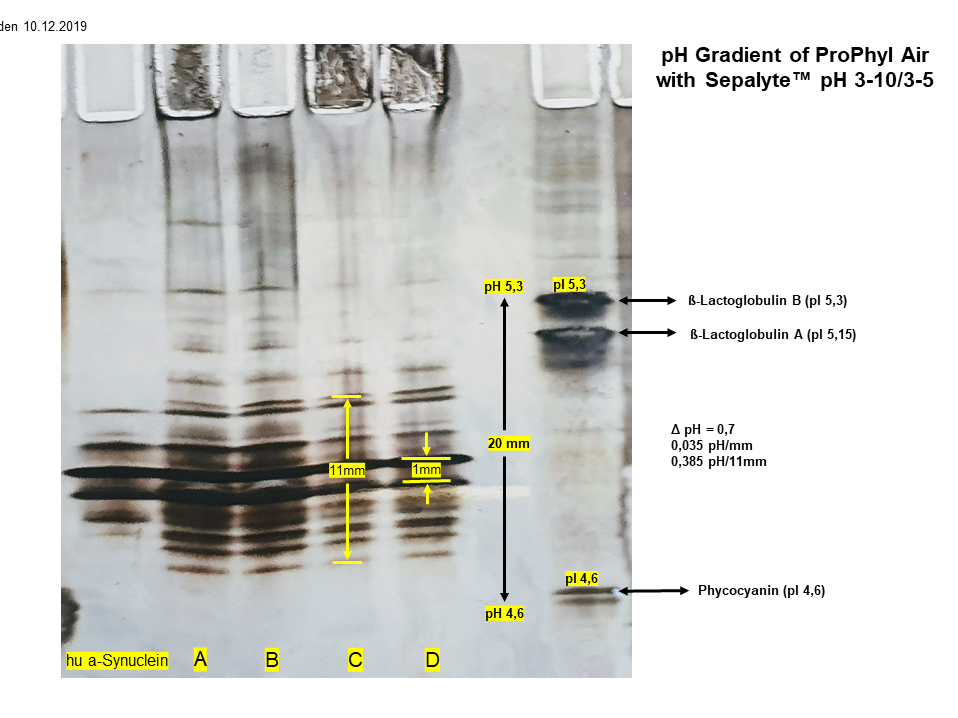
alpha-synuclein in different fibril stages
Fig.1 left
Sample A,B,C,D
α-synuclein in different aggregation stages of fibril production (intermediate fibrils).
Sample C + D
Large amounts of insoluble aggregated fibrils can be found on the application point (below).
Sample S
monomeric α-synuclein
Fig.1 right
Sample A,B,C,D,S
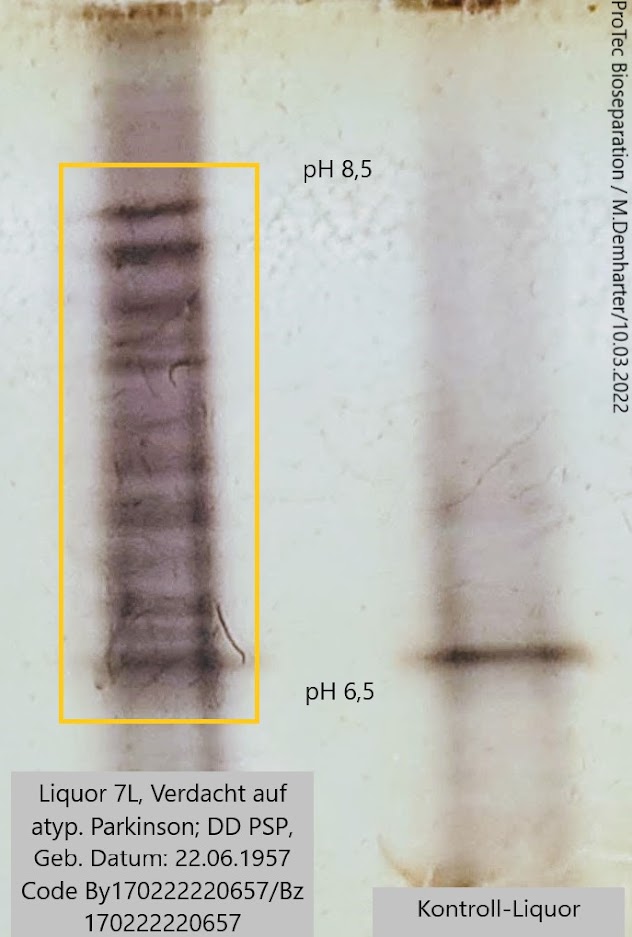
previously unknown proteins in the high pH range (> pH 8,5)
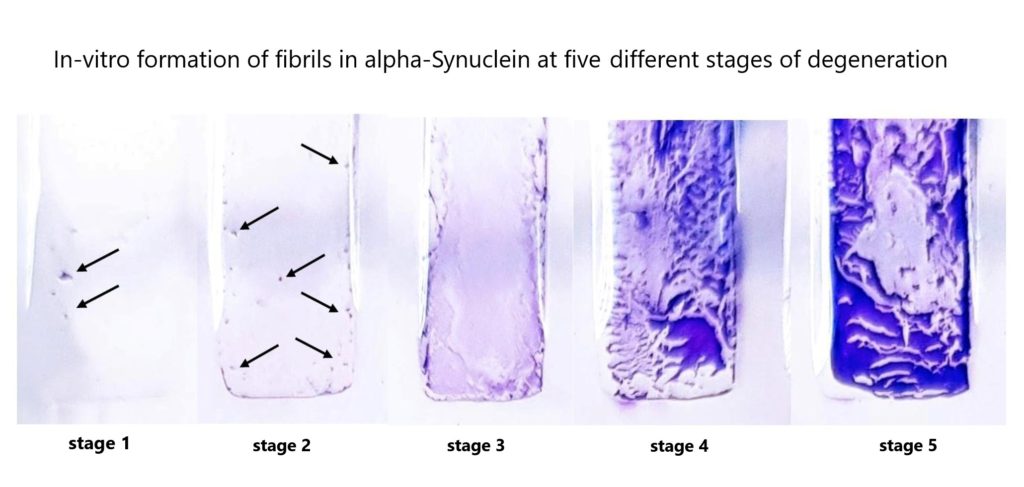
The formation of intermediate fibril aggregation shown in the fig. 1 left could be an indication that one or more misfolding isoforms of the α-synuclein shown in the IEF (fig. 2) could be the cause of the production of macromolecular amyloid fibrils the leads to Parkinson’s. When α-syn is incubated in vitro at a high concentration under shaking conditions, it undergoes a conformational change and turns into fibrils within a few days.
In contrast, little or no fibrils form at a lower α-syn concentration without shaking, and the protein needs more time to assemble. Studies indicate that α-syn exists in secretory vesicles of neurons and multifarious biological fluids such as CSF and plasma, implying that α-syn may be secreted via exocytosis. Interestingly, they found CSF α-synuclein levels at 12 months were lower in people with PD treated with dopamine replacement therapy, especially dopamine agonists.
α-synuclein is expressed principally in the nervous system, but it is also produced in other tissues, including the skin. In the brain, the protein is primarily neuronal, but it is also present in glia. Neuronal α-synuclein is concentrated in the presynaptic nerve terminals, interacts with plasma membrane phospholipids, and is also present in the nuclei and mitochondria. At least six synuclein bands of unknown origin (fig. 2). The most common isoform is a 140 amino acid-long transcript. Other isoforms are α-synuclein-126, lacking residues 41-54; and α-synuclein-112, which lacks residues 103-130. α-synuclein’s physiological role is poorly understood, but the protein has been implicated in regulating dopamine release and transport, synaptic vesicle clustering, and functioning as a SNARE-complex chaperone.
Sample A,B,C,D
α-synuclein in different stages of fibril aggregation (intermediate fibrils).
Sample hu-α-synuclein
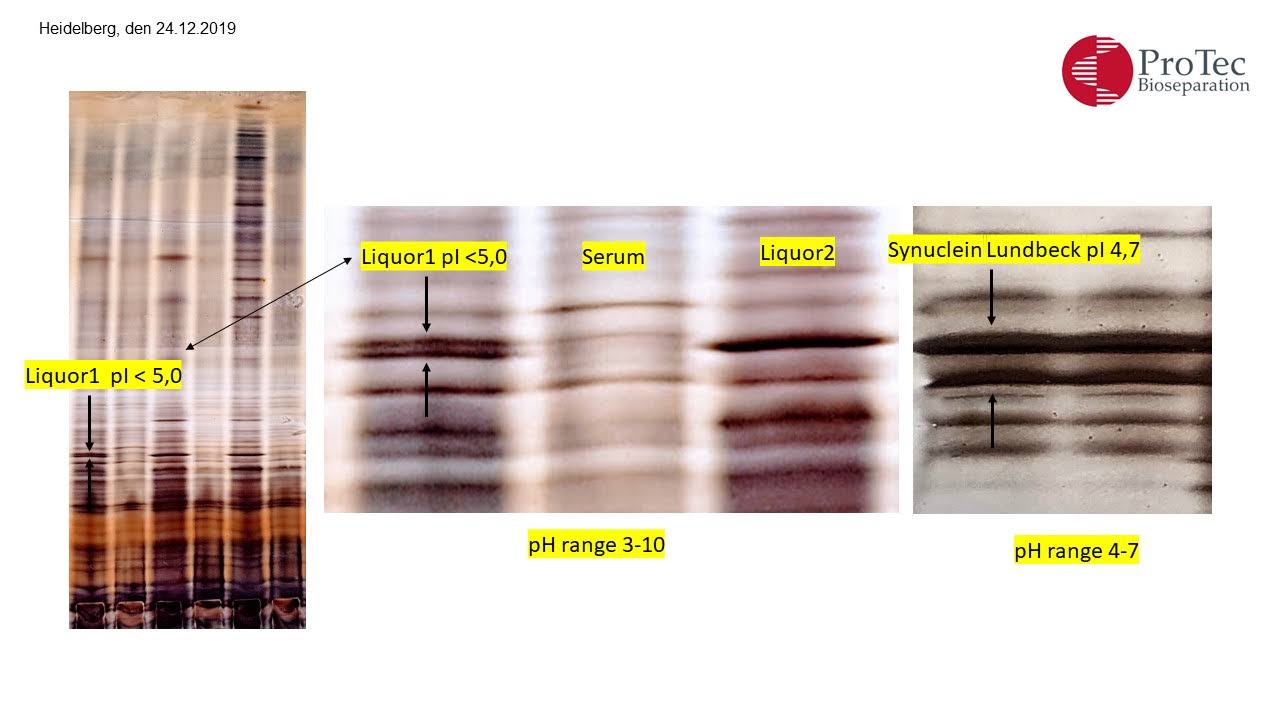
Human monomeric synuclein checked with FPLC and SDS electrophoresis (figure 2+4). Several bands were identified in the IEF with ProPhyl Air that could result from the protein fragmentation into subunits. This finding is new and needs further investigation.
α-synuclein fibrils are a major component of the intracellular Lewy bodies that are associated with Parkinson’s disease, Lewy body dementia, and multiple system atrophy (see below)
ProPhyl Air made a decisive contribution to the discovery of α-synuclein in cerebrospinal fluid using the isoelectric focusing method