
Melon
PGM – Phosphoglucomutase
16 % v/v glycerol
Bring 200 grams (= 160 ml) of glycerol (q.c.w. Sigma G-7757) and 800 ml distilled water in a beaker. Mix well and bring to a final volume of 1000 ml with distilled water. Store in the refrigerator.
Sepalyte pH 3-6 (Cat. No.42006 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Acrylamide/ bis acrylamide 29 : 1 solution
Ready to hand acrylamide solution (q.c.w. Sigma A-3574). Store in refrigerator.
0.1N Sodium hydroxide
4.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 1000 ml distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature
0,1% w/v riboflavin
0.05 g riboflavin (q.c.w. Sigma R-0508) in 50 ml 0.1 N sodium hydroxide.
Store in the refrigerator for up to 2 weeks.
10% w/v Ammonium persulfate
Dissolve 0.5 grams in 5 ml dH2O. Store in refrigerator.
PGM extraction solution 0.5 % v/v pH 3-10 Sepalyte (Cat.No.: 42008 ProTec Bioseparation)
2.5 ml Sepalyte (3- 10) in 500 ml distilled water. Add a trace (tip of a small spatula) of Orange G (q.c.w. Sigma O-1625). Store in refrigerator
0.2 M Tris-HCl pH 8.0
Bring 48.4 g Tris (q.c.w. Sigma T-8524) and 0.8 g EDTA (q.c.w. Sigma E-5134) in 1600 ml distilled water. Stir until fully dissolved. Adjust the pH to 8.0 with (± 24 ml) concentrated HCl (q.c.w. BDH 28507). Bring to a final volume of 2000 ml with distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature.
4 M magnesium chloride
Bring 203-g magnesium chloride hexahydrate (q.c.w. Sigma M-0250) and 150 ml distilled water in a beaker. Stir until fully dissolved and bring to a final volume of 250 ml. Store at room temperature.
Glucose-6-phosphate-dehydrogenase (Sigma G 7750)
Dissolve 2000 U in 10 ml water and divide into 0.4 ml in eppendorf vials, equal to 80 U per vial. Store the vials in the freezer.
2 % v/v Acetic acid solution
Bring 20 ml of acetic acid 100% (q.c.w. BDH 27013) in 1000 ml distilled water. Store at room temperature.
Gel preparation
9.5 ml 16 % glycerol
2.0 ml Acrylamide/bis 29:1solution
1.5 ml Sepalyte pH 3-6
0.065 ml 0.1 % w/v riboflavin
0.012 ml TEMED
0.035 ml 10% ammonium persulfate
Add the above reagents and swirl to mix. Pour the gel according to the flap technique and allow polymerizing for at least 4 hours under light. Store the gels in a sealed bag in the refrigerator for up to 2 weeks.
Sample preparation
Single cotyledon punches (using the cork borer # 3, diameter 7 mm) from soil or towel germinated seeds (6-8) days old are placed into a 96 well microplate. Samples are frozen for 2 hours or overnight. To each well, between 200 and 225µl PGM- extraction solution is added. See to it that the confetti-like punchouts are on the bottom of the wells. Terminator for 3 minutes (termination should be started immediately after adding extraction buffer while samples are still frozen) and then centrifuged for 10 minutes at 3000 –3500 rpm. at 10° C (or at room temperature).
Electrophoresis
Turn the cooling supply on and set it at a temperature of ± 15°C. Remove the gel from the glass plates. Clean the back of the gel with methanol/ethanol. Place the gel onto the cooling plate with several ml of water. The gel can be divided into four parts. Space the electrodes evenly across the gel, alternating cathode (black electrode) and anode (red electrode), and then place directly onto the gel.
Power settings are for one gel (double the mA and Watts when running two gels).
Prefocusing
Run 1: 600 V–60 mA–10 W–100 V/h
Sample application
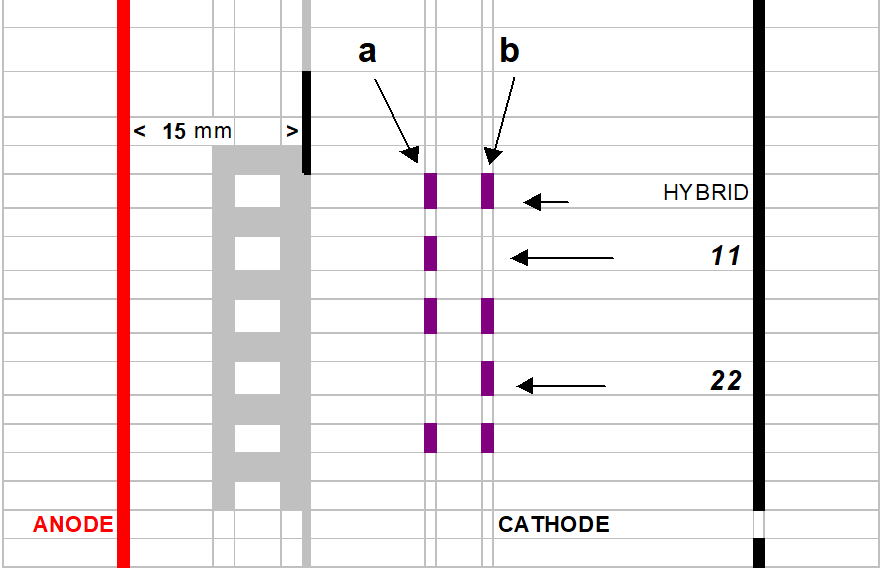
After the prefocusing step, the 52 templates are positioned ± 20 mm from the cathode (black electrode). See cartoon image section “gel interpretation 9.1.6 “ Each sample well is filled with 15 µl of supernatant.
Focusing
Run 2: 300 V–60 mA–10 W–100 V/h
Run 3: 1000 V–60 mA–20 W–1500 V/h
After the gel has finished running, remove the gel from the cooling plate and place into an appropriate staining tray.
PGM staining
120 ml 0.2M Tris / HCl pH 8.0
0.2 ml 4 M magnesium chloride
0.50 g α-D- glucose-1-phosphate disodium salt (Sigma G 7000) (add last)
0.05 g NADP (q.c.w. Sigma N-3886)
0.05 g MTT (q.c.w. Sigma M-2128)
0.01 g PMS (q.c.w. Sigma P-9625) (tip of the small spatula)
80 Units Glucose-6-phosphate-dehydrogenase (q.c.w. Sigma G 7750) (add last)
Heat up the Tris-HCl solution to ± 37° C. Add NADP, PMS, MTT, and 4M MgCl and mix until fully dissolved. Just prior to pouring the stain over the gel add the α-D-glucose -1-phosphate disodium salt (Sigma G 7000) and the glucose-6-phosphate dehydrogenase (q.c.w. Sigma G 7750). Stain the gel at ± 37°C or at room temperature until the bands can be clearly visualized. Remove the stain and destain the gel in a 2 % glacial acetic acid solution for about 10 minutes and rinse with distilled water for an additional 10 minutes. The gel can be air-dried.
Gel interpretation
No international enzyme classification is used.
The neatest band of interest from the anode is genotyped “11”
The farthest band of interest from the anode is genotyped “22”.
The hybrid is genotyped “1/2” in case that the female is 11 and the male 22.
The hybrid is genotyped “2/1” in case that the female is 22 and the male 11
ProTec Bioseparation (Protein Electrophoresis)
