Melon
PRS II – (Protein from Seed)
16 % v/v glycerol
Bring 200 grams (= 160 ml) of glycerol (q.c.w. Sigma G-7757) and 800 ml distilled water in a beaker. Mix well and bring to a final volume of 1000 ml with distilled water. Store in the refrigerator.
Sepalyte pH 3-10 (Cat.No. 42008 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Sepalyte pH 3-6 (Cat.No. 42006 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Acrylamide/bis 29 : 1 solution
Ready to hand acrylamide solution (q.c.w. Sigma A-3574). Store in refrigerator.
0.1N Sodium hydroxide
4.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 1000 ml distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature
0,1% w/v riboflavin
0.05 g riboflavin (q.c.w. Sigma R-0508) in 50 ml 0.1 N sodium hydroxide.
Store in the refrigerator for up to 2 weeks.
10% w/v ammonium persulfate
0.5 g ammonium persulfate (q.c.w. Sigma A- 3678) in 5.0 ml distilled water.
Store in refrigerator for one week
4 N Sodium hydroxide
16.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 100 ml distilled water.
Store in the refrigerator.
Tetra Methyl Urea (TMU) extraction solution
Bring together 7.0 ml of tetramethyl urea (q.c.w. Sigma T- 3875), 9.0 ml ethylene glycol (q.c.w. Sigma E-9129) and 30 ml of distilled water. Mix well before use. Add a trace (tip of a small spatula) of Orange G (q.c.w. Sigma O-1625). Do not store.
Trichloroacetic acid ( TCA) 20 % w/v
Bring 400 g of trichloroacetic acid (q.c.w. Sigma T-4885) and 1500 ml water in a beaker. Stir until fully dissolved. Bring to a final volume of 2000 ml with distilled water.
Store at room temperature.
Coomassie stock solution
Bring 1.0 g brilliant blue (q.c.w. Sigma B-7920) in 200 ml ethanol 96%. Stir until fully dissolved. Add 250-ml water and 50 ml 100 % acetic acid (q.c.w. BDH27013) to the solution. Mix well before use. Store at room temperature.
Destain solution
Bring together 200-ml acetic acid 100% (q.c.w. BDH 27013), 800 ml ethanol 96% (or methanol 100%) and 1000 ml distilled water. Store at room temperature
Coomassie working solution
Bring together 50 ml fresh stock solution and 250 ml destain solution. Do not store.
TEMED
Ready to use TEMED Sigma ( T-9281).
Gel preparation
9.5 ml 16 % glycerol
2.0 ml Acrylamide/bis 29:1solution
0.3 ml Sepalyte pH 3-6
0.7 ml Sepalyte pH 3-10
0.065 ml 0.1 % w/v riboflavin
0.012 ml TEMED
0.035 ml 10% ammonium persulfate
Add the above reagents and swirl to mix. Pour the gel according to the flap technique and allow polymerizing for at least 4 hours under light. Store the gels in a sealed bag in the refrigerator for up to 2 weeks.
Sample preparation
Single seeds punches are placed into a 96 well microplate. To each well, 200 ul of TMU extraction solution is added. The seeds are homogenized with the Terminator and centrifuged for 10 minutes at 3000 rpm. At 10° C (or at room temperature). Repeat centrifugation if time permits.
Electrophoresis
Turn the cooling supply on and set at a temperature of ± 15°C. Remove the gel from the glass plates. Clean the back of the gel with methanol/ethanol. Place the gel onto the cooling plate with several ml of water. The gel can be divided into three parts. Space the electrodes evenly across the gel, alternating cathode (black electrode) and anode (red electrode) and then place directly onto the gel.
Power settings are for one gel (double the mA and Watts when running two gels).
Prefocusing
Run 1: 600 V–60 mA–12 W–75 V/h
Sample application
After the prefocusing step, the 52 templates are positioned ± 15 mm from the anode (red electrode). See cartoon image section “gel interpretation 9.1.6“ Each sample well is filled with 15 µl of supernatant.
Focusing
Run 2: 200 V–60 mA–12 W–50 V/h
Run 3: 1000 V–60 mA–12 W–1000 V/h
After the gel has finished running, remove the gel from the cooling plate and place into an appropriate staining tray.
Coomassie staining
Pour on the gel ±300-ml of 20% TCA solution. Let the proteins precipitate for 5 minutes. After the 5 minutes of fixation, swirl the tray for 15 minutes.
Rinse off the TCA, using dH2O.
Add ± 300 ml Coomassie working solution to the gel. Stain until blue bands can be clearly visualized.
Rinse off the blue stain using dH2O, destain (if necessary) with the destaining solution. Gel can be air-dried.
Gel interpretation
Interpret the bands of interest. The protein pattern is multiple banded.
The nearest band of interest from the anode is genotyped “11”.
The farthest band of interest from the anode is genotyped “ 22”.
The hybrid is genotyped “1/2” in case that the female is 11 and the male 22.
The hybrid is genotyped “2/1” in case that the female is 22 and the male 11.
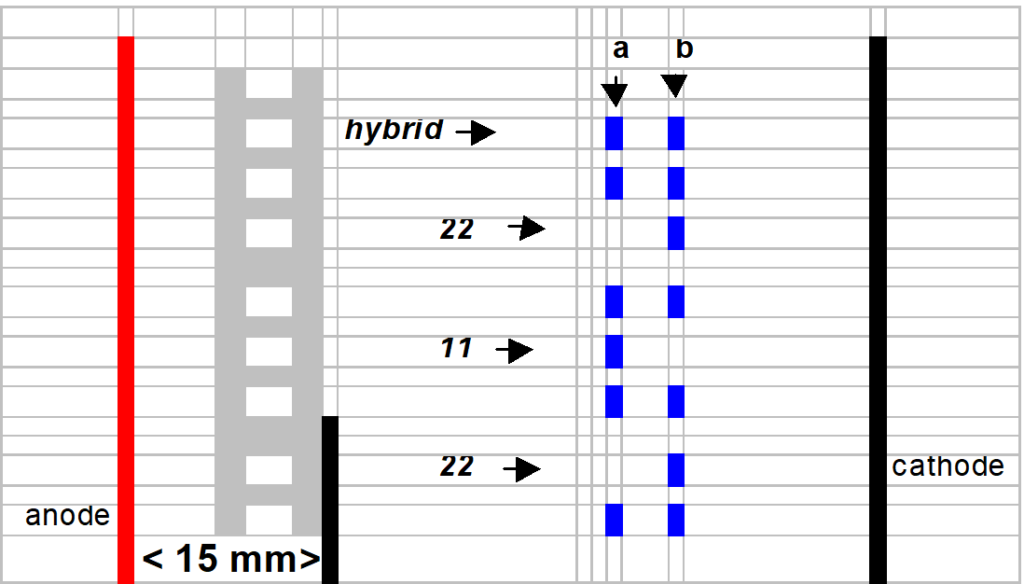
This is an illustrative cartoon image and does not picture the real PRS profile.
ProTec Bioseparation (Protein Electrophoresis)

