Protein Electrophoresis Protocols for Seed Testing
1. Preparation of Horizontal Thin-layer Acrylamide Gels for Isoelectric Focusing
Thin horizontal gels are easily poured between 2 glass plates.
(How do I make a horizontal IEF gel? Check it out now! https://vimeo.com/user72932045)
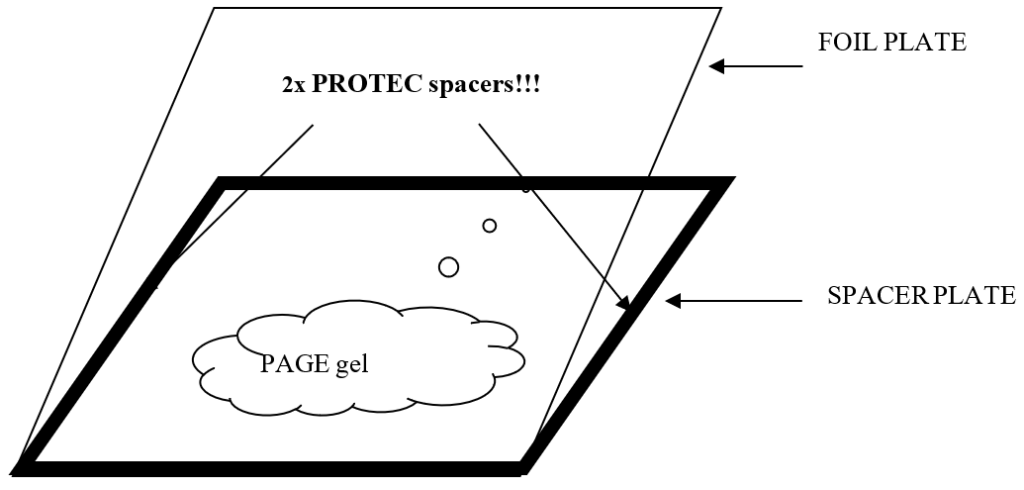
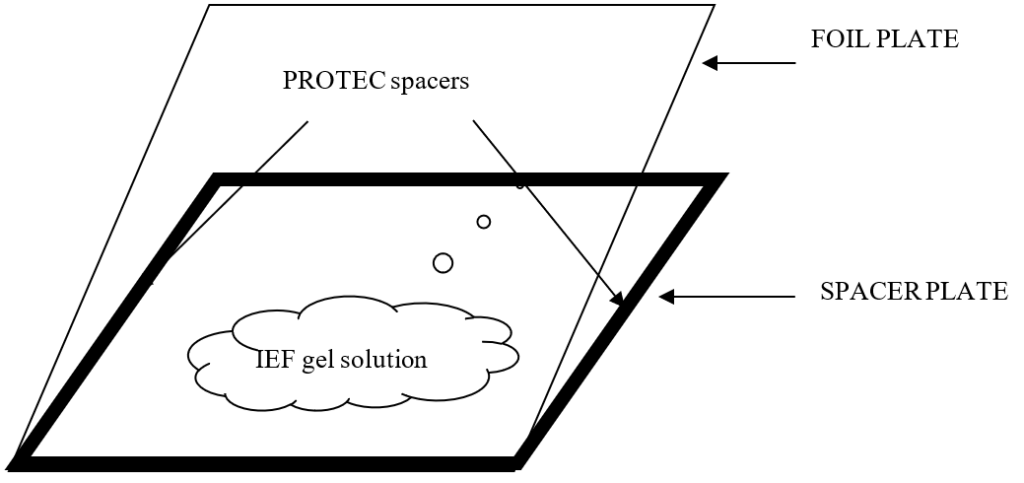
A SPACER PLATE can be made by taking a clean glass plate (2 x 193 x 265 mm) and taping all four sides with PROTEC spacer tape (270 µm thick) as shown in Figure 1.
Protein electrophoresis SPACER PLATE
All four sides of the glass plate are taped with PROTEC tape leaving some small open space at the edges to makes sure that no air bubbles are trapped between the glass plates. Samples are applied onto the gel using a sample applicator strip.
Clean glass plates need to be used so that the tapes does not come off when the SPACER PLATES are re-used dozens of times. If needed, clean the glass plates thoroughly with acetone and/or alcohol.
After applying the tape, leave the plate overnight at 60 C in an oven. This will assure that all glue solute will vaporize and that the tape will stick to the glass plate also when washing many times.
Treat the glass plate with chemicals (chemical or polymer-based repellant) to assure that the acrylamide will not stick to the glass plate. The SPACER PLATE is then ready for use. Blue Slick (SERVA) treated glass plates can be re-used many times (up to 10 times), before another treatment is necessary. Between the different uses of the SPACER PLATE, it only needs a quick rinse with water. It is recommended to keep the plates dust-free.
A FOIL PLATE is made by putting a special polyester foil GelStick http://www.protec-biosep.de/isoelectric-focusing/gelstick/ (treated to bind polyacrylamide) – also called gel backing – onto a clean glass plate with several ml of water in between. After putting the foil on the glass plate, roll onto the foil to remove excess of water and any air bubbles. Wipe the foil and edges dry with a paper tissue. The FOIL PLATE is now ready for use.
Use a bench with ample space to pour the gels. The bench must be completely flat to assure easy gel pouring. Place the SPACER PLATE (spacers on top) and the FOIL PLATE (foil on top) next to one another on top of filter paper. The filter paper will soak up any gel solution that might be spilled from the gel. Non-polymerized acrylamide is highly toxic so see to it that after pouring the filter paper is removed and the bench is cleaned.
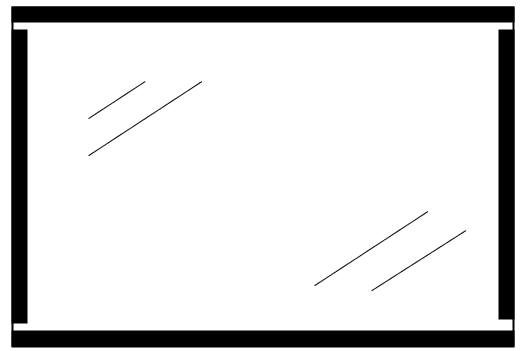
When the Plates are on the bench, make the appropriate gel solution by mixing the different components and pour the solution onto the SPACER PLATE. Then, the FOIL PLATE must be brought with one of the edges towards the edge of the SPACER PLATE in the way as shown in Figure 2.
Figure 2. Image showing the start of the gel pouring process. By slowly lowering the FOIL PLATE towards the SPACER PLATE, the gel solution will be trapped between the 2 plates and form a thin gel.
The FOIL PLATE must be lowered slowly towards the other plate so that the gel solution spreads evenly between the plates and taking care not to trap air bubbles between the plates. After flapping the plates, allow the solution to polymerize. When using ammonium persulfate as a polymerizing agent, this will take between 5 and 15 minutes. Polymerization using riboflavin can best be done overnight. After polymerization, the gel can be stored in a sealed plastic bag (with a piece of moist tissue paper) for up to 2 weeks.
2. Preparation of Horizontal Thin-layer Acrylamide Gels for Native Protein Electrophoresis.
The FOIL PLATE must be lowered slowly towards the other plate so that the gel solution spreads evenly between the plates and taking care not to trap air bubbles between the plates. After flapping the plates, allow the solution to polymerize. When using ammonium persulfate as a polymerizing agent, this will take between 5 and 15 minutes. Polymerization using riboflavin can best be done overnight. After polymerization, the gel can be stored in a sealed plastic bag (with a piece of moist tissue paper) for up to 2 weeks.
Horizontal thin-layer acrylamide gels for native protein electrophoresis can be made in a similar way as IEF gels. The gels are easily poured between 2 glass plates, the SPACER PLATE and the FOIL PLATE as described above under 1.
The principal difference is that the gel is twice as thick as an IEF gel. Thus, 2 layers of spacer tape must be used to make the SPACER PLATE.
And, of course, the gel solution is different as described.