New-Discovery in Alzheimer’s research with the help of ProPhyl Air– NEW
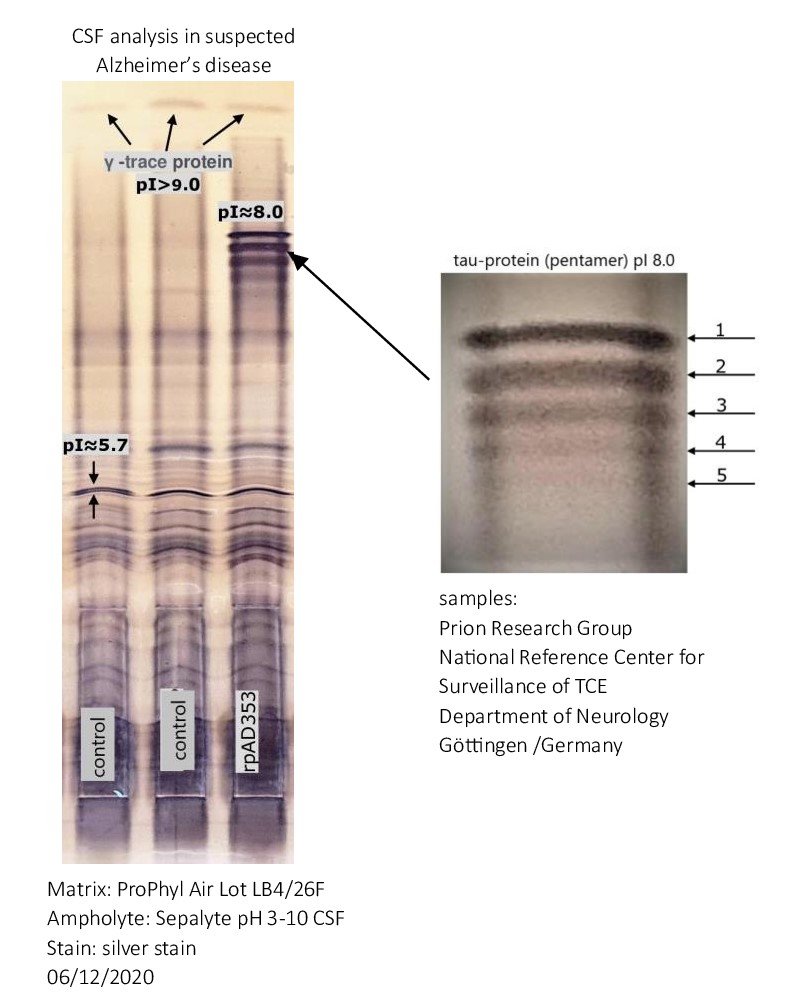
Alzheimer’s disease: Detection of oligomerized Tau protein using hydrophobic interaction isoelectric focusing (HIEF)
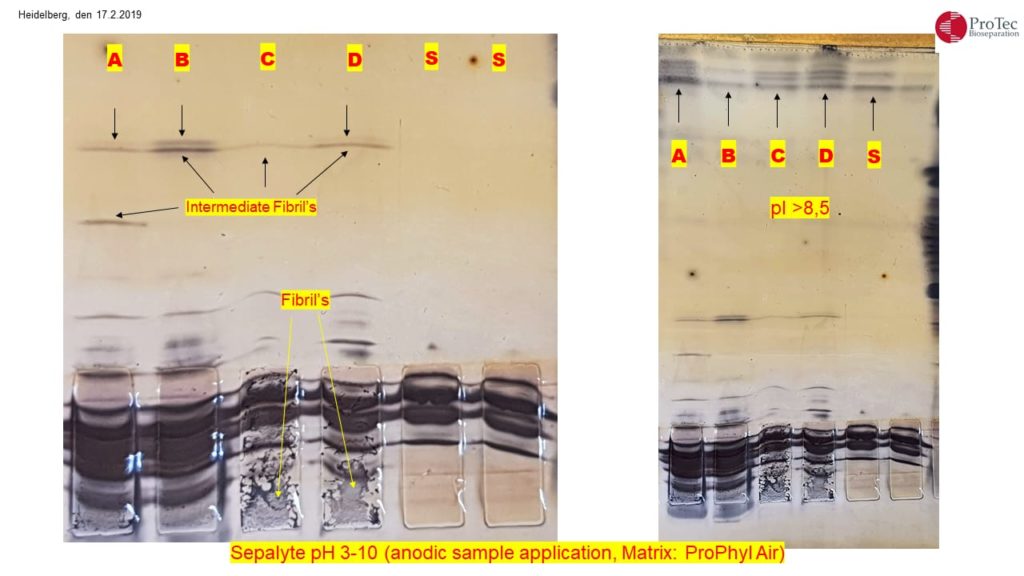
The detection of five bands with an isoelectric point (pI) of approximately 8.0 in the cerebrospinal fluid of an Alzheimer’s patient suggests the presence of isoforms of tau protein. Tau protein is known to have multiple isoforms, and alterations in its structure and phosphorylation state are associated with neurodegenerative conditions such as Alzheimer’s disease. The distinct bands likely represent different phosphorylation states or modifications of tau protein.
When the tau protein undergoes phosphorylation, it can lead to changes in its structure and function. Tau is a microtubule-associated protein that plays a crucial role in stabilizing microtubules in neurons. Phosphorylation is a process where phosphate groups are added to the protein.
Different phosphorylation states of tau can affect its ability to bind to microtubules. In its normal, non-phosphorylated state, tau promotes microtubule stability. However, excessive phosphorylation can lead to a detachment of tau from microtubules, causing them to disassemble. This disruption of microtubule stability is associated with the formation of neurofibrillary tangles, a characteristic feature of several neurodegenerative disorders, including Alzheimer’s disease.
Moreover, abnormal tau phosphorylation has been linked to the development of tauopathies, where tau aggregates in the form of insoluble fibrils, contributing to neuronal dysfunction and cell death. The specific consequences of tau phosphorylation depend on the sites and extent of phosphorylation, highlighting the intricate regulatory mechanisms involved in maintaining proper neuronal function.
CSF analysis in suspected Alzheimer’s disease and other dementia using the new one-dimensional hydrophobic interaction isoelectric focusing method
About Alzheimer’s
Alzheimer’s disease is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills and, eventually, the ability to carry out the simplest tasks. In most people with the disease those with the late-onset type symptoms first appear in their mid-60s. Early-onset Alzheimer’s occurs between a person’s 30s and mid-60s and is very rare. Alzheimer’s disease is the most common cause of dementia among older adults.
The disease is named after Dr. Alois Alzheimer.
In 1906, Dr. Alzheimer noticed changes in the brain tissue of a woman who had died of an unusual mental illness. Her symptoms included memory loss, language problems, and unpredictable behavior. After she died, he examined her brain and found many abnormal clumps (now called amyloid plaques) and tangled bundles of fibers (now called neurofibrillary, or tau, tangles).
These plaques and tangles in the brain are still considered some of the main features of Alzheimer’s disease. Another feature is the loss of connections between nerve cells (neurons) in the brain. Neurons transmit messages between different parts of the brain, and from the brain to muscles and organs in the body. Many other complex brain changes are thought to play a role in Alzheimer’s, too.
This damage initially appears to take place in the hippocampus, the part of the brain essential in forming memories. As neurons die, additional parts of the brain are affected. By the final stage of Alzheimer’s, damage is widespread, and brain tissue has shrunk significantly
Brain imaging links Alzheimer’s decline to tau protein
Tau is better marker of progression to Alzheimer’s disease than amyloid beta
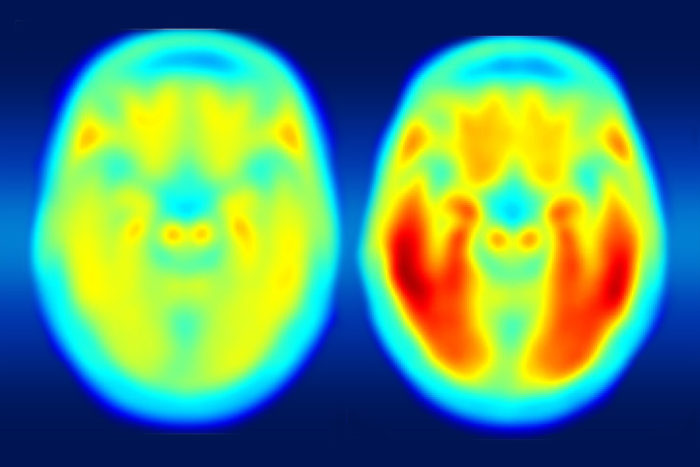
A study using a new PET imaging agent shows that measures of tau protein in the brain more closely track cognitive decline due to Alzheimer’s disease compared with long-studied measures of amyloid-beta. More red color indicates more tau protein. The image on the left shows the average tau accumulation in the brains of cognitively normal people, averaged over many individuals. The image on the right shows the average amount of tau buildup in the brains of multiple people with mild Alzheimer’s symptoms. Scanning multiple individuals shows that the intensity of tau deposits correlates with the severity of cognitive dysfunction. (Image: Matthew R. Brier)
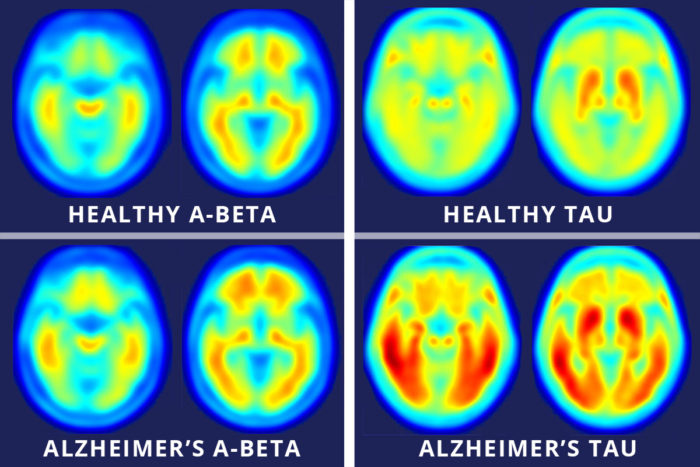
Images across the top show compilations of PET brain scans of people who are cognitively normal. Images across the bottom show compilations of PET brain scans of patients with symptoms of mild Alzheimer’s disease. The difference between scans of healthy people and scans of patients with mild Alzheimer’s disease is much more apparent in the images that measure tau (right four images), suggesting tau protein buildup in the brain is a better marker of Alzheimer’s disease symptoms than the long-studied amyloid-beta buildup (left four images). (Image: Matthew R. Brier)
Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, Owen C, Aldea P, Su Y, Hassenstab J, Cairns NJ, Holtzman DM, Fagan AM, Morris JC, Benzinger TLS, Ances BM. Tau and Ab imaging , CSF measures, and Cognition in Alzheimer’s disease. Science Translational Medicine. May 11, 2016.
“Our work and that of others has shown that elevated levels of amyloid beta are the earliest markers of developing Alzheimer’s disease, but in the earliest stages of Alzheimer’s disease, even with amyloid buildup, many patients are cognitively normal, meaning their memory and thought processes are still intact. What we suspect is that amyloid changes first and then tau, and it’s the combination of both that tips the patient from being asymptomatic to showing mild cognitive impairment.”
CSF examination service
Free examination of sample material (CSF) with the condition that an anamnesis (potentially medically relevant information) is provided.
Contact information: m.demharter@protec-biosep.de
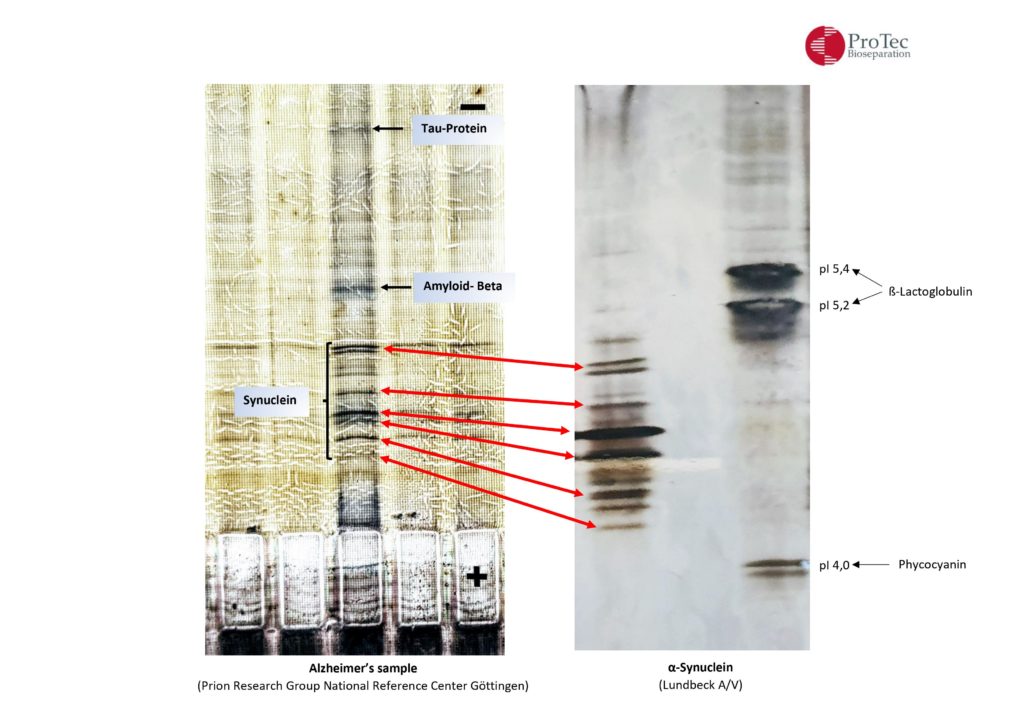
| α-synuclein |
| H. Lundbeck A/S, Ottiliavej 9, 2500 Valby, Denmark |
| CSF Alzheimer’s |
| Prion Research Group, National Reference Center for Surveillance of TSE, Neurology, Göttingen, Germany |
Alzheimer`s and Parkinson`s
AEP protease acts on amyloid, tau and now α-synuclein
Alzheimer’s disease and Parkinson’s disease are not the same. They affect different regions of the brain and have distinct genetic and environmental risk factors. But at the biochemical level, these two neurodegenerative diseases start to look similar. In both Alzheimer’s (AD) and Parkinson’s (PD), a sticky protein forms toxic clumps in brain cells. In AD, the troublemaker inside cells is called tau, making up neurofibrillary tangles. In PD, the sticky protein is α-synuclein, forming Lewy bodies.
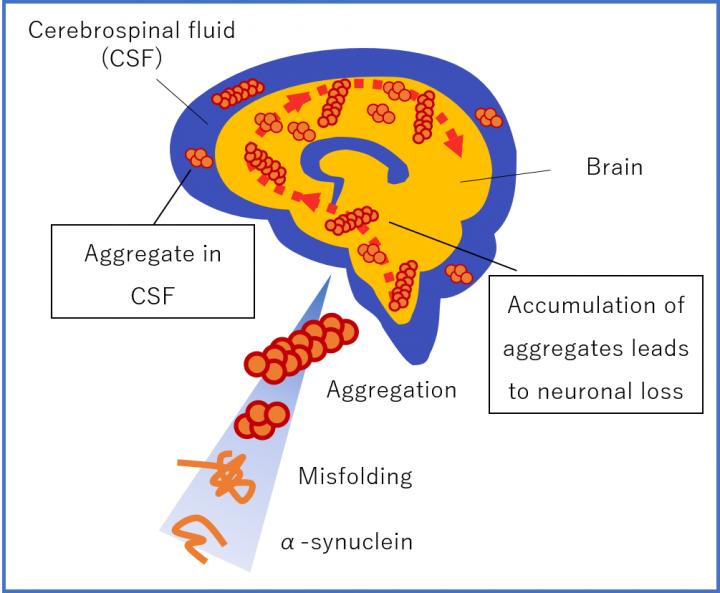
Why does α-synuclein play such an important role in Alzheimer’s
α-synuclein aggregation are the most potent neurotoxic species responsible for the pathogenesis of PD and AD. Misfolded protein aggregates are pathologic hallmarks for many neurodegenerative disorders, including Alzheimer’s, Parkinson’s and prion disease. Protein aggregates are typically β-sheet rich oligomers or fibrils that occur as intracellular or extracellular deposits in the central nervous system.
In patients with neurodegenerative disease, two or more protein aggregates commonly co-exist, for example α-synuclein and amyloid β
in dementia with Lewy bodies and tau and amyloid β in Alzheimer’s disease. Consistent with these observations, a patient was recently reported that had suffered from a rapidly progressive dementia with multiple aggregated proteins existing in the brain, including prions, tau, α-synuclein and amyloid β, which may suggest a general disturbance in protein folding or an initial protein aggregate catalyzing the aggregation of additional proteins.