Melon
PEPLA – (Peptidase with leucylalanine as substrat)
16 % v/v glycerol
Bring 200 grams (= 160 ml) of glycerol (q.c.w. Sigma G-7757) and 800 ml distilled water in a beaker. Mix well and bring to a final volume of 1000 ml with distilled water. Store in the refrigerator.
Sepalyte pH 4-5 (Cat.No. 42009 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Sepalyte pH 3-7 (Cat.No. 42007 ProTec Bioseparation)
Ready to hand Sepalyte solutions. Store in refrigerator
Acrylamide/ bis acrylamide 29 : 1 solution
Ready to hand acrylamide solution (q.c.w. Sigma A-3574). Store in refrigerator.
0.1N Sodium hydroxide
4.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 1000 ml distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature
0,1% w/v riboflavin
0.05 g riboflavin (q.c.w. Sigma R-0508) in 50 ml 0.1 N sodium hydroxide.
Store in the refrigerator for up to 2 weeks.
10% w/v ammonium persulfate
0.5 g ammonium persulfate (q.c.w. Sigma A- 3678) in 5.0 ml distilled water.
Store in refrigerator for one week
PEPLA extraction solution 0.5% Sepalyte (pH 3-6 Cat. No.42006)
2.5 ml Sepalyte (pH 3-6 Cat. No.42006) in 500 ml distilled water. Add a trace (tip of a small spatula) of Orange G (q.c.w. Sigma O-1625). Store in refrigerator
0.05 Tris-HCl pH 7.0
Bring 12.0 g Tris (q.c.w. Sigma T-8524) in 1600 ml distilled water. Stir until fully dissolved. Adjust the pH to 7.0 with concentrated HCl (q.c.w. BDH 28507). Bring to a final volume of 2000 ml with distilled water. Add a trace (tip of a small spatula) of sodium azide (q.c.w. Sigma S-8032). Store at room temperature.
4 M magnesium chloride
Bring 203-g magnesium chloride hexahydrate (q.c.w. Sigma M-0250) and 150 ml distilled water in a beaker. Stir until fully dissolved and bring to a final volume of 250 ml. Store at room temperature.
2 % v/v acetic acid solution
Bring 20 ml of acetic acid 100% (q.c.w. BDH 27013) in 1000 ml distilled water. Store at room temperature.
4 N Sodium hydroxide
16.0 g sodium hydroxide (q.c.w. Sigma S-8045) in 100 ml distilled water.
Store in the refrigerator.
TEMED
Ready to use TEMED Sigma ( T-9281).
Gel preparation
9.5 ml 16 % glycerol
2.0 ml Acrylamide/bis 29:1solution
0.5 ml Sepalyte pH 3-7
0.5 ml Sepalyte pH 4-5
0.065 ml 0.1 % w/v riboflavin
0.012 ml TEMED
0.035 ml 10% ammonium persulfate
Add the above reagents and swirl to mix. Pour the gel according to the flap technique and allow polymerizing for at least 4 hours under a light. Store the gels in a sealed bag in the refrigerator for up to 2 weeks.
Sample preparation
Single cotyledon punches (using the cork borer # 3, diameter 7 mm) from soil or towel germinated seeds (6-8) days old are placed into a 96 well microplate and frozen for two hours or better overnight. Tissue extraction should be done while tissue is still frozen. To each well, 100 µl cold PEPLA extraction solution is added. See to it that the confetti-like punchouts are on the bottom of the wells. The samples are homogenized using the Terminator for 3 minutes. After homogenization, an additional 120 µl PEPLA- extraction solution is added and mixed. Mix and centrifuge for 10 min. at 3000 rpm.
Electrophoresis
Turn the cooling supply on and set it at a temperature of ± 15°C. Remove the gel from the glass plates. Clean the back of the gel with methanol/ethanol. Place the gel onto the cooling plate with several ml of water. The gel can be divided into three parts. Space the electrodes evenly across the gel, alternating cathode (black electrode) and anode (red electrode), and then place directly onto the gel.
Power settings are for one gel (double the mA and Watts when running two gels).
Prefocusing
Run 1: 600 V–60 mA–10 W–100 V/h
Sample application
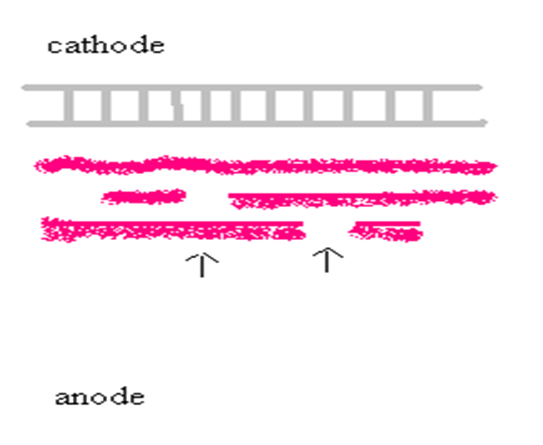
After the prefocusing step, the 52 templates are positioned ± 12 mm from the cathode (black electrode). See cartoon image section “gel interpretation 9.1.6 “ Each sample well is filled with 15 µl of supernatant.
Focusing
Run 2: 200 V–60 mA–10 W–100 V/h
Run 3: 1000 V–60 mA–10 W–1500 V/h
After the gel has finished running, remove the gel from the cooling plate and place it into an appropriate staining tray.
PEPLA staining
Solution 1
Leucine alanine 0.08g (Sigma L-9250)
Peroxidase 0.04g (Sigma P-8125)
Amino Acid Oxidase 0.08g (Sigma V-7000)
0.05M Tris-HCl pH 7.0 50ml
Solution 2
Agarose 1.6g (Sigma A-0169)
0.05M Tris-HCl pH 7.0 50ml
Solution 3
3-amino-9-Ethylcarbazole 0.1g (Acros 14787-5000)
N, N dimethylformamide 12ml (Acros 11622-0010)
Chemicals in solution 2 should be weighed up in a beaker that is 3-4 times the size of the solutions volume. Chemicals in solution 1 should be weighed up in a beaker that is large enough to hold the combined solutions of 1,2, and 3.
Heat solution 2 to boiling (about 1 min) in the microwave.
Then cool to approximately 70°C.
While solution 2 is cooling, dissolve solutions 1 and 3 separately.
When solutions 1 and 3 are dissolved mix them together.
When solution 2 is cooled to the appropriate temperature it is then added to the combined solution of 1 and 3. This solution is then slowly poured onto the gel. The bands will occur on the cathodal half of the separation field, so make sure that section of the gel is covered by the overlay. Gels will be read the next day.
Gel interpretation
No international enzyme classification is used.
The first band of interest from the anode is genotyped “11”
The furthest band of interest from the anode is genotyped “ 22”
The hybrid is genotyped “1/2” in case that the female is 11 and the male 22.
The hybrid is genotyped “2/1” in case that the female is 22 and the male 11.
ProTec Bioseparation (Protein Electrophoresis)

